
Platinum: The Valuable Strong element
What is Platinum?
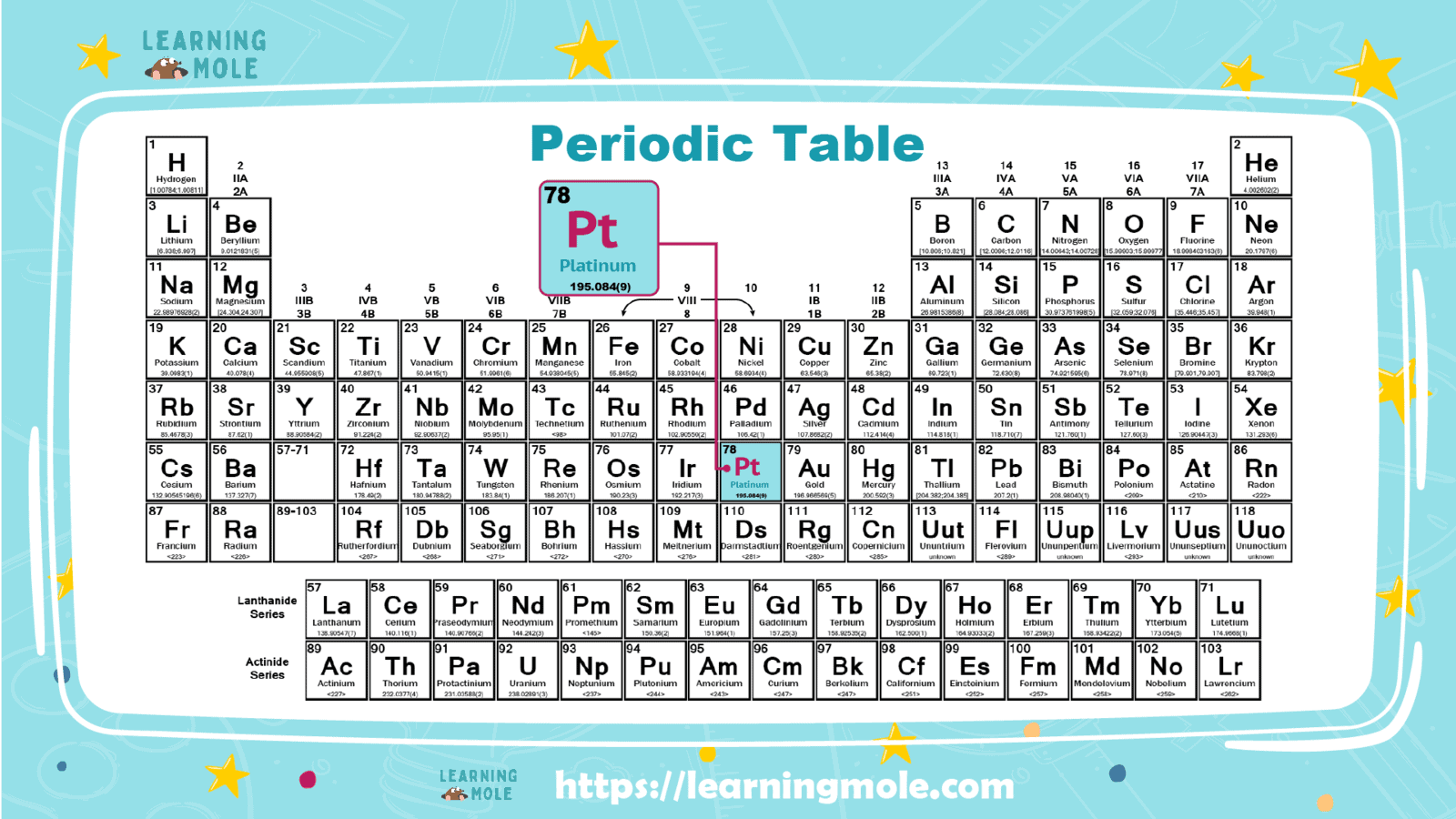
It is one of the important chemical elements, widely spread and used, symbolized by the chemical symbol “Pt”; its atomic number equals “78”, it is located in the periodic table within the elements of the tenth group and the sixth period, and it follows in its classification to the transition metals.
The reason behind naming platinum with this name is due to one of the Spanish terms known as “plata”, which is a word that means little silver. It may be considered one of the strongest metals, especially iron, in addition to its flexibility, which is similar to that of gold.
It has been called white gold since ancient times. This is due to the remarkable similarity between it and the gold element, in addition to its high flexibility, as the name white gold is given to that alloy that consists of a mixture of different metals that were produced as a substitute for the platinum element during the gold-making operations.
It is one of the strongest, most vital and valuable and most precious metals. This is due to the fact that during the discovery of this metal, large quantities of it were found in a group of deposits of platinum ore, which are located in particular in Latin America.
The value of this element was not known at the beginning of its discovery, Which made its material value and cost somewhat low. With the increase in research and studies on this element, it was found that it has different characteristics from the rest of the elements. In addition to its multiple uses, its material and economic value increased.
Locations
It is like many other elements that cannot be found freely or singly in nature, as it appears associated with many elements and metals, and one of the most important elements to which this element is associated and which has been included in one group known as the platinum group is the element palladium, osmium, iridium, in addition to its link to the element ruthenium and rhodium.
In addition, It has spread in many regions and countries of the world, as it is spread in large quantities in Russia, Indonesia and Brazil, and its prosperity has increased in South America, Greece and Italy.
It is also possible to find the platinum element in the form of granules characterized by their small size or in the form of relatively large blocks characterized by their high weight, which makes it able to form a group of ores.
Among the most famous of these ores is saberlite, an ore in which platinum is associated with arsenic, which leads to the formation of platinum arsenide, in addition to cuprite, copper, iron and nickel ore.
Discovery and naming
The first to discover platinum
The first complete characterization of platinum was by a Spanish military commander and man named Don Antonio de Ulloa (1716-1795) during his service in South America from (1735-1746). He collected samples of platinum and finally wrote a report on it, describing its mining or extraction as well as its uses.
He was given platinum discovery credit on the basis of the report he wrote. Information on this element spread throughout Europe, where scientists were fascinated by its physical properties, Not only for its beauty when looking at it but also for its resistance to corrosion (rust).
Many people consider it to be used in making jewellery and artefacts such as gold and silver. The demand for it began to increase, and this led to the so-called platinum era “Spain”.
A reference to platinum was found in the writings of the Italian physicist Scholar and poet Julius Caesar Scaliger in the period between 1484-1558. Scaliger saw platinum while visiting Central America in 1557.
He referred to it as a solid metal, and the citizens were involved in working with it, but the Spaniards did not. The metal was called Platina (meaning little silver), and this name was given by the citizens.
It was obtained during the mining of silver and gold, and the citizens thought it had no use and thought it was a troublesome thing.
Physical properties of platinum
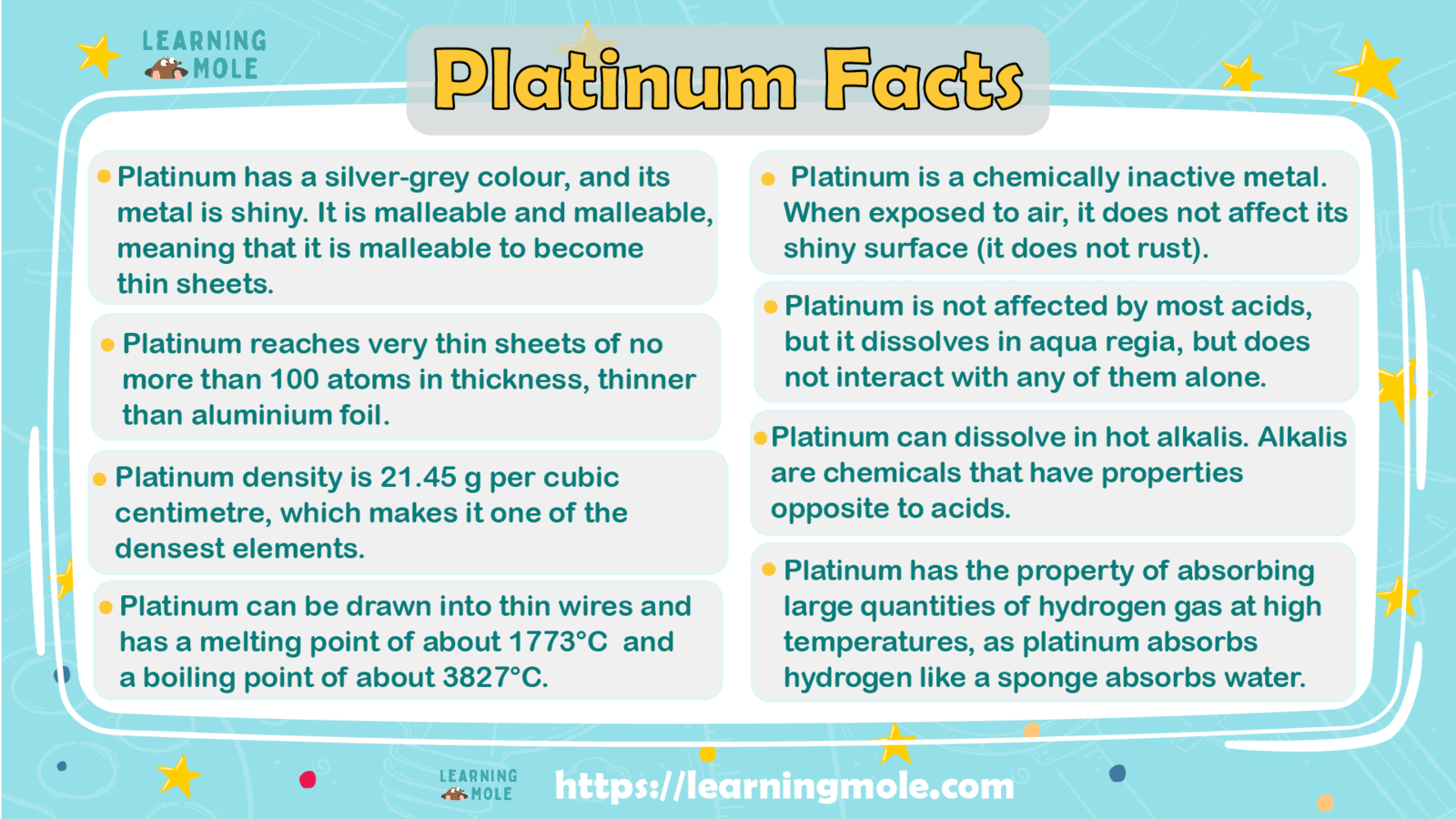
Platinum has a silver-grey colour, and its metal is shiny. It is malleable and malleable, meaning that it is malleable to become thin sheets, and with more methods, it reaches very thin sheets of no more than 100 atoms in thickness, thinner than aluminum foil.

A malleable means that it can be drawn into thin wires and has a melting point of about 1773°C (3223°F) and a boiling point of about 3827°C (6921°F). Its density is 21.45 g per cubic centimetre, which makes it one of the densest elements.
Chemical properties of platinum
It is a chemically inactive metal. When exposed to air, it does not affect its shiny surface (it does not rust) and is not affected by most acids, but it dissolves in aqua regia (a mixture of hydraulic acid and nitric acid) but does not interact with any of them alone.
It can also dissolve in hot alkalis. Alkalis are chemicals that have properties opposite to acids. Sodium hydroxide (caustic) and lime water are examples of alkalis.
It has the property of absorbing large quantities of hydrogen gas at high temperatures, as platinum absorbs hydrogen like a sponge absorbs water.
Its presence in nature
The metals that make up the platinum group exist together, which creates difficulties when extracting them and trying to separate them from the group of other metals.
It is not like gold, these minerals are not present in large quantities so that they can be extracted, but it is usually extracted as a secondary metal when extracting other minerals such as: –(copper-nickel).
It is one of the rarest elements. Its abundance can be estimated at 0.01 parts per million of the Earth’s crust. South Africa is the largest supplier of platinum.
In 1996, South Africa produced 117 tons of platinum. The second country in terms of production in the world is Canada, as it produced 8,260 tons in 1996. The United States comes as a large producer, as most of the production is from the Steel-water mines in Montana.
Platinum isotopes
- There are six natural isotopes of platinum (190, 192, 194, 195, 196, 198) of all of them. There is only isotope 190 that has radioactivity.
- Isotopes have two or more forms of the element.
- Isotopes differ from one to the other according to their mass number. The mass number is written to the right of the element’s name, and the mass number represents the number of protons in addition to the number of neutrons in the nucleus of an atom of the element.
- The number of protons determines the element, but the number of neutrons in an atom of any element varies (each variation is an isotope).
- Radioactive isotopes of platinum have also been produced industrially. These isotopes are produced when very fine particles are directed at atoms. These particles stick to the atoms and make them radioactive.
There are no radioisotopes of platinum for any commercial use.
Platinum extraction
Separating platinum from other platinum minerals is the primary challenge to obtaining pure platinum. The first step in the separation process is to dissolve the mixture in water aqua regia.
As platinum dissolves in aqua regia, while other platinum minerals do not dissolve, platinum metal can be removed from aqua regia, where the platinum is in a spongy form of black platinum powder,
The last stage is heating that powder at very high temperatures, where the it melts, producing a pure metal.
Platinum compounds that have important commercial use are relatively few. As mentioned above, pacemakers made of platinum protect the device from corrosion and damage from the effect of acids inside the body.
Impact on health
Platinum in its dusty form and some platinum compounds have a moderate effect on health. If inhaled, it makes a person sneeze and causes inflammation in the nose and shortness of breath. If it is spilt on the skin, a rash and inflammation of the skin will occur.
Benefits of the platinum metal
The platinum group is considered one of the precious elements, and this is due to it being relatively inert, as it does not combine or interact with most elements or compounds, which makes it have some uses as it enters, for example, into the manufacture of laboratory equipment, because it will not interact with the materials that will come into contact with those devices.
It is used as a catalyst, and the catalyst is known as a substance that accelerates a chemical reaction without any change in it. For example, the catalytic converter in a car’s exhaust system can contain platinum 95.

Platinum Uses
The most common use among people is as jewellery.
This is due to the fact that it is a (hard, attractive, corrosion-resistant) metal, as it is used in the manufacture of: (bracelets, earrings, clasp pins used for decoration, watch bracelets, and other types of jewellery).
The most important use of platinum is not in the jewellery industry.
Where it is used in the manufacture of catalysts; An example of this is in the modern petroleum industry. Where the crude oil in the ground is processed before it is converted into gasoline, fuel oil, and other petroleum products, the petroleum molecules must be broken apart, rearranged and then reattached together into a new molecular arrangement and shape. It is one of the most widely used catalysts for this purpose.
It is used as a catalyst for the manufacture of final compounds such as fertilizers, plastics, medicines and pharmaceutical products, and dozens of other products that are emerging daily, such as the manufacture of nitric acid. Nitric acid is used to manufacture ammonia, and fertilizers are made from it.
The ideal use for such element would likely be as a catalyst in automobiles, As all modern cars have a catalytic converter in the exhaust system. It is a device that helps gasoline burn almost completely and thus reduces the number of pollutants emitted into the air. The vast majority contain platinum or any metal from the platinum group.
And we can summarize some others:
- It is widely used in the manufacture of many different metal alloys.
- Sometimes it is involved in the manufacture of many thin sheets and wires that are used in many fields.
- It is used in the manufacture of some parts and engines of cars.
- It can be used in the process of dismantling some parts of the oil.
- It can be used in the manufacture of certain types of jewellery and ornaments.
- It is used in the manufacture of some surgical instruments and equipment and in the manufacture of some laboratory instruments.
- It can be used to treat some diseases, such as cancer.
- It is used in the manufacture of some electrical wires and connectors.
- It goes into many different coating processes.
- It can be used in the manufacture of utensils, flasks, and containers that are used in chemical laboratories.
- It is used in the manufacture of certain types of magnets, especially those found in watches, batteries, and some medical devices.
- Malleable, malleable and ductile.
- It can be used in the manufacture of some types of fertilizers and plastics.
- It is often used in the production of gasoline and fuel cells.
- It has been used since ancient times in the manufacture of many decorative materials and pieces.
- A cylindrical piece of this element can be used in kilogram measurements.
- Enters the manufacture of some musical instruments.
Other uses for platinum
Platinum is also used in pickup trucks as certain types of spark plugs contain platinum; In general, in the United States of America, the largest and only use of platinum is in cars and trucks.
Many of its uses are based on its chemical activity. Some people cannot live without an artificial heart, as pacemakers are implanted in their chests, which is a device that ensures that the pattern of artificial heartbeats works regularly. Corrosion or its malfunction, which makes it necessary to add it as a substitute in the body.
It is also used in trace amounts in alloys. For example, cobalt is alloyed with platinum to make strong magnets. Melting and mixing two or more metals results in an alloy. The properties of this mixture are different from the properties of each individual metal. Platinum-cobalt magnets are among the strongest magnets known.
Other Resources:
If you enjoyed learning about this brilliant metal, why not check out more fantastic facts about other metals like: Gold, Silver, Iron, Manganese, Mercury, Zinc and Copper.
Why not subscribe to our LearningMole Library for as little as £1.99 per month to access over 1300 fun educational videos.


Leave a Reply