
Mercury: Many debates about the valuable Element
What is Mercury?
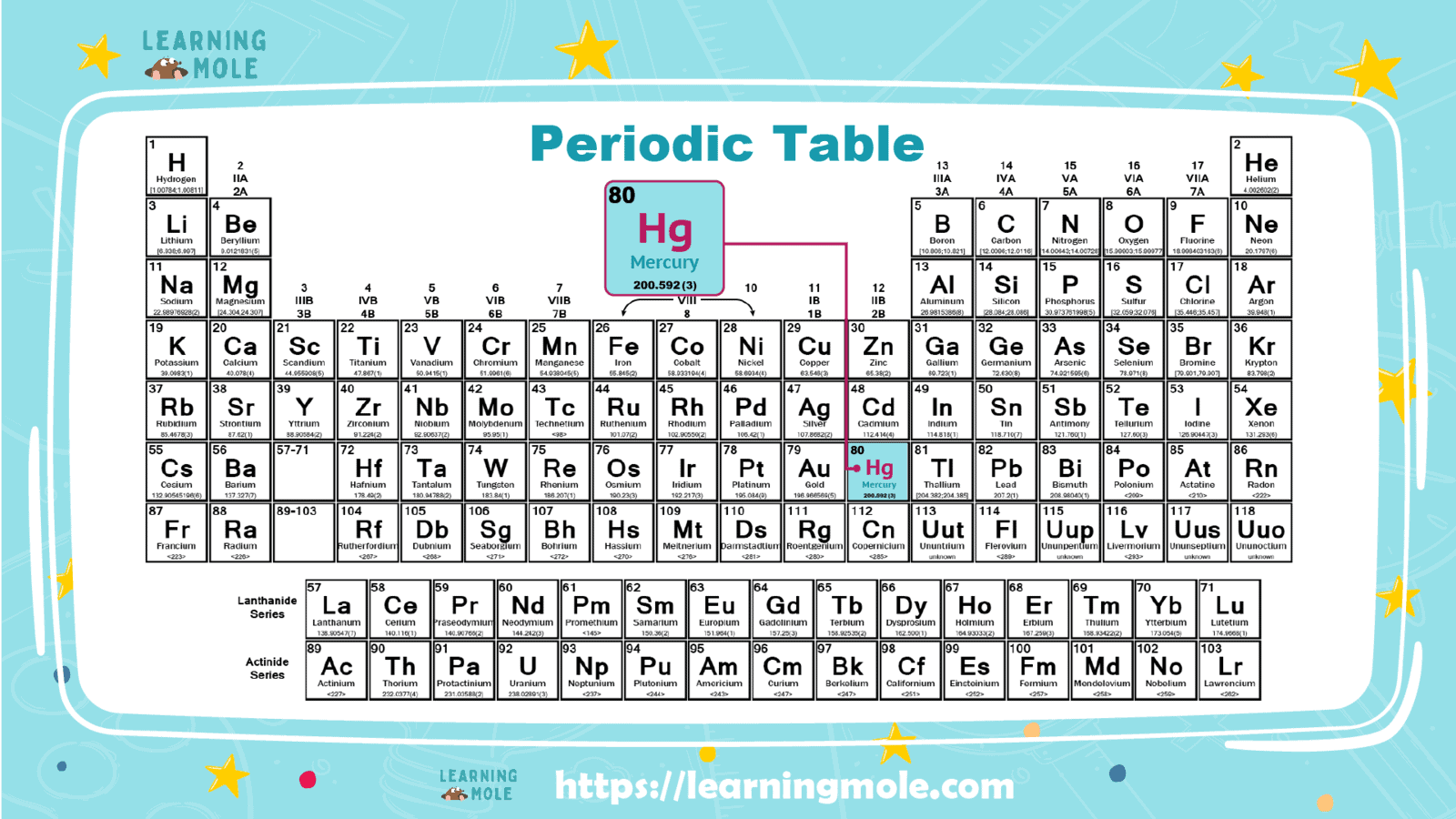
Mercury is one of the important chemical elements. It has its symbol, “Hg”, and its atomic number is “80”; it cannot exist freely in nature; Due to its ability to bind with a number of different elements and form its own compounds, the most important of which is mercury sulfide.
The raw material for mercury is found in nature through a number of natural factors. Such as volcanoes, floods, and rock carving operations, in addition to the continuous use of coal and cement industry, and the most famous type of mercury is Russian mercury, which is considered an industrial radioactive isotope, and Pharaonic mercury, and this mercury is found naturally.
As the most important sources of mercury, they are the pollutants emitted from power plants, especially those that depend mainly on burning waste and coal. This leads to pollution of air, water, and everything that exists on the surface of the earth, including plants and animals, in addition to humans who may be exposed to poisoning as a result of this process.
It has great harm to the waters of the seas, oceans and rivers. Where this damage is in the form of large pollution that results from nature itself without the role of industrial residues in it; In addition to that, mercury evaporates and spreads with the air in multiple and broad areas until it is eventually deposited and concentrated in the seas and oceans.
It is possible for mercury to enter the human body in a number of ways, either by inhaling it or eating it, and this may reach the human body through wounds, causing damage to the nerves, liver and kidneys, in addition to many symptoms.
Cinnabar is the main raw mineral for mercury; It is a soft mineral whose colour ranges from red to brown, and it is extracted from the ground through the mining process.
It is one of the only elements that exist in a liquid state under standard temperatures and pressures, leading to the formation of round beads.
It can be present in the earth’s crust, but in very small quantities compared to other elements, and it may also be found in several ores such as cinnabar ore; This is done by heating this ore until the mercury is obtained, but in a completely pure form.
Exposure to mercury in large quantities leads to poisoning, which results in several symptoms such as irritation of the lungs, nausea and vomiting, in addition to high blood pressure and rash, in addition to a feeling of numbness in most parts of the body.
It is one of the metals that can be easily destroyed and disposed of, and It can be recycled and used for various purposes without the need for mining.

Mercury discovery
The name mercury is derived from the Roman god Mercury, the intelligent messenger of the gods; This is because the ancients used this name for the element known since prehistoric times, as the symbol Hg is derived from the Greek word (hydrargyrum) which means “liquid silver” or “fast silver”.
It was known to the ancient Chinese and Hindus and was found in Egyptian tombs 3,500 years old, as mercury is usually not found alone in nature, and it is obtained mainly from the mineral cinnabar with the chemical formula (HgS).
Mercury metal is the only common metallic liquid at ordinary temperatures, as it rarely appears alone in nature, and Spain and Italy produce about 50% of the global supply of the metal, as the commercial unit for handling mercury is the “flask” which weighs 76 pounds Mercury metal is obtained by heating cinnabar in a stream of air and by process of vapour condensation.
Mercury metal is a silvery-white heavy metal, and it is a somewhat weak conductor of heat when compared to other metals. It is a fair conductor of electricity, and it easily forms alloys with many different metals, such as gold, silver, and tin, which are called amalgams.
It is a toxic element that can enter the body through the respiratory tract, the digestive tract, or directly through the skin, as it builds up in the body and eventually leads to severe illness or death.
Spain and Italy produce about half of the supply world of mercury.
The importance of Mercury in practical life
- It has been used mainly for medicinal purposes, although its toxicity was discovered early on.
- It was used in the past to extract gold and silver from its raw materials due to its ease of solubility.
- It is used in the manufacture of chlorine and caustic soda (sodium hydroxide) by electrolysis of salt solutions.
- It is used in the preparation of agricultural and industrial pesticides and pharmaceutical preparations.
- It is included in the manufacture of ultraviolet lamps and fluorescent lamps.
- The electrical conductivity of mercury is such that it is helpful for use in relays and exceptionally closed switches.
- It is used as a coolant in nuclear reactors and in manufacturing barometers and manometers.
- It is used primarily in many electronic and electrical applications and in the manufacture of various chemicals.
- It is the main component of amalgam fillings in dentistry, although there is controversy about its health effects.
- It forms part of a secondary electrode as a substitute for an electrode. The standard for hydrogen in the fields of electro-chemistry.
- It plays the role of a catalyst in some chemical reactions.
- It forms the organic compound thiomersal, used as a preservative in vaccines.
- It was used in the past for preservation.
- On wood, making silver mirrors, and some types of paints, mercury compounds were also used in the manufacture of antidepressants, laxatives, and anti-psychotics, in addition to antiseptics, eye drops, nasal sprays and some diuretics.
Physical Properties
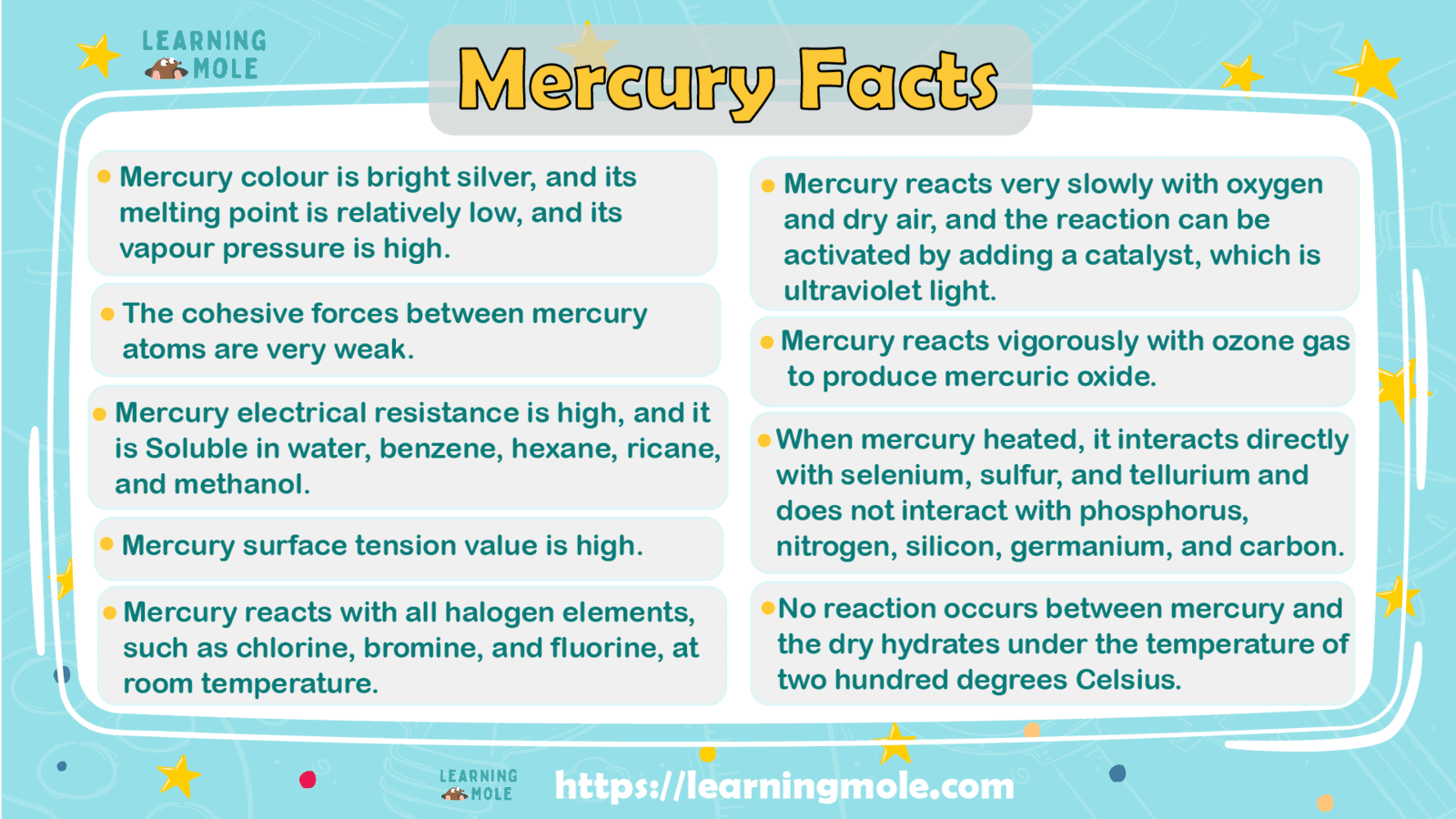
- Its colour is bright silver.
- Its melting point is relatively low, and its vapour pressure is high.
- The cohesive forces between its atoms are very weak.
- Its electrical resistance is high.
- Soluble in water, benzene, hexane, ricane, and methanol.
- The value of its surface tension is high, as the value of its surface tension is six times greater than the surface tension of water.
Chemical Properties
- It reacts with all halogen elements, such as chlorine, bromine, and fluorine, at room temperature.
- It reacts very slowly with oxygen and dry air, and the reaction can be activated by adding a catalyst, which is ultraviolet light.
- It reacts vigorously with ozone gas to produce mercuric oxide.
- When heated, it interacts directly with selenium, sulfur, and tellurium and does not interact with phosphorus, nitrogen, silicon, germanium, and carbon.
- No reaction occurs between it and the dry hydrates under the temperature of two hundred degrees Celsius, such as ammonia hydrogen.
- There is no reaction between it and hydrogen chloride acid and dilute sulfuric acid, and the reaction can occur by increasing the concentration of the acid.
- No reaction occurs between it and phosphorous acid, while it interacts with nitrogenous acid.
- Strong interactions occur between it and ammonia solutions.
- Mercury salt is used as a catalyst for many reactions.
Types of Mercury
Red Mercury:
It is made of organic materials and vital tissues, and it is sometimes made from natural materials such as gold, through exposure to a long period of time from radiation; in addition to that, it may be extracted from the ground, especially from the craters of volcanoes after their eruption, but the quantities of its extraction are relatively small; Which makes it expensive because it is difficult to extract.
Red mercury is used in the manufacture of fusion bombs. It is used instead of fissile fuel as a primitive detonator. It is also involved in facilitating uranium enrichment. In order to benefit from it and use it for many military purposes, it prevents the spread of weapons of mass destruction, and red mercury may enter the Egyptian mummies industry since ancient times.
This type can be defined as an explosive material that is used to manufacture many nuclear weapons and atomic bombs. This mercury is considered one of the most dangerous and harmful materials, especially if it is widely spread in many parts of the world.
White mercury:
A type of mercury that is most widely used by humans. It is included in the manufacture of thermometers that are used at the present time. It is characterized by its rapid expansion when temperatures rise, but if temperatures drop, it will shrink. This can be obtained from drug stores.
White mercury is found in many precious rocks. Whether these rocks were stones produced by the volcano ejecting them during its eruption, or stones coming from outside the planet, which appear in the form of meteors of different characteristics, this may be mercury at the beginning of its formation is white, gradually changing its colour as a result of its continuous exposure to sunlight.
Black mercury:
This type of mercury is considered one of the ancient mythical minerals. Man tries to search for and extract it permanently. Its presence represents a great treasure, as it gives its owner great energy.
It was discovered by many scientists conducting several experiments and research until they reached it. It is used in the manufacture of nuclear bombs. It is characterized by its high density compared to other elements, in addition to being stronger than titanium and uranium, which makes it an expensive item.
Radioactive mercury
This type of mercury has a different composition and is involved in several industries, the most important of which is the nuclear industry. This type of mercury is present in the earth’s crust in small quantities.
Mercury compounds
Mercury oxide: This compound is produced by heating mercury in the air at specific temperatures. It has an attractive red colour. It decomposes when heated to give both oxygen and mercury.
Mercury chloride: It is formed by heating and dissolving mercury oxide when it is placed in concentrated hydrochloric acid, or by preparing and heating a mixture consisting of table salt and mercury sulfate.
Calomel mercury chloride: It is characterized by its white colour, its ability to dissolve in water is low, and it can be obtained by heating and mixing both mercury sulfate and table salt with each other.
Mercury acetate: A highly toxic compound that is formed by analyzing the oxidation of mercury and putting it in acetic acid. This compound is used in the preparation of a number of organic mercury compounds.
Colours of Mercury
1- Silver mercury
This type is the most widespread and is called elemental mercury or metallic mercury. It is the widespread form of silver. It is found in nature in silver or white. It is in a liquid state at room temperature, but it evaporates when exposed to air. Mercury is used in several uses, the most famous of which is the manufacture of thermometers.
2 – Red mercury
Mercury may exist in red in nature, or it may be formed from the interaction of mercury with some elements such as sulfur and chlorine to create another type of mercury called inorganic mercury, which is a dangerous compound that may lead to poisoning or burns to anyone who touches it.
3 – Black mercury
Black mercury is the same composition as red mercury, as it consists of the interaction of mercury with sulfur and the formation of a mercury sulfide compound. Still, when red mercury is heated to a temperature exceeding 250°, red mercury turns into black mercury.
Mercury Toxicity
Mercury is a metal with a particular characteristic, that of being liquid at room temperature. This compound can be found in organic form (Hg), inorganic (Hg+, Hg2+) and also in the form of vapour in the elemental state (Hg0). The organic form of mercury is more toxic than the inorganic form.
Mercury is used as an anti-mould in paints, in the plastics industry as a catalyst and by dentists as a dental amalgam. In the past, it was also used in thermometers and the conservation of vaccines.
In the food sector, cereals and fish are the foods most polluted by mercury.
Mercury, like other metals, has the ability to bind with the -SH groups of microsomal and mitochondrial proteins and enzymes. This mechanism of action causes both acute and chronic toxicity, non-specific damage and cell death. In the case of acute intoxication, the effects observed are pneumonia, with neurological symptoms at the CNS and gastrointestinal tract levels.
In chronic toxicity, the effects observed include tremors, forms of hallucinations, renal damage, neuro-toxicity at the level of the cerebral cortex (one of the areas of the brain responsible for cognitive activity) and of the cerebellum (partly used in the motor activity), movement alterations, muscle weakness, loss of vision and hearing, death.
Methyl-mercury (MeHg) is a compound that derives from the methylation of mercury and is very harmful to our body because it has a strong ability to bind to the -SH groups of the tubules of the cytoskeleton, causing serious damage at this level and during neuronal migration and mitotic division of cells, resulting in cell apoptosis.
Thanks to considerable laboratory studies, it has been possible to ascertain how methyl-mercury administered to female rats during pregnancy causes damage to the cytoskeleton and principles of cellular apoptosis in the unborn. Methyl-mercury, therefore, does not cause structural damage (the organogenesis has already occurred) but causes cellular alterations that will be appreciated above all in adulthood.
Why it’s a health hazard and how to avoid it
The fight against mercury protects our health. Because of that chemical element, there are very high risks for our bodies, as well as the stability of the ecosystem. We can ingest it by feeding ourselves, especially with fish, or using beauty products. And we’ve introduced it into our mouths in the past through dental amalgam. With this intention,UNEP, the United Nations organization for the protection of the environment, has launched an information campaign on a global scale to make public opinion more aware.
Excessive exposure can, in fact, cause various ailments, from “simple” headaches or nausea, to the onset of serious diseases such as cancer. According to the World Health Organization (WHO), two groups of people are particularly at risk: unborn children whose mothers have high levels of mercury in their blood, as well as those who are regularly exposed to the agent, such as fishermen.
Diet is a means by which mercury can be introduced into the body. Seafood is the main source of protein for more than three billion people worldwide. Larger fish, such as sharks, swordfish, tuna, and marlin, tend to be particularly high in mercury as the chemical “builds up” in the food chain. People who consume very large amounts of seafood run the risk of ingesting a large amount.
Cosmetics: watch out for the purchase
Another danger is represented by cosmetic products. Mercury is also found in beauty products, especially creams, but also make-up and eye cleansers. Of course, many countries have introduced stringent regulations forbidding it from cosmetics. But many others have yet to do so, and these products are readily available online. In this case, the security tool is that of conscious purchase.
More specifically, mercury poses a threat to the health of mine workers. “Mercury poisoning also represents a serious and direct threat to the health of the 12-15 million people who work in the sector worldwide”. But there is a further effect. Artisanal and small-scale mining emitted about 800 tonnes of mercury into the air, about 38% of the global total, and also released about 1,200 tonnes of mercury into land and water. The reduction of fossil sources is also important on this point. Coal burning not only contributes to air pollution and the climate crisis but is also a major source of anthropogenic mercury emissions.
Mercury in dental amalgam
Mercury has been rampant every time we go to the dentist. For more than a century, mercury has been a primary component of dental amalgam., the mixture used to fill the cavities of patients’ teeth. But “while amalgam probably poses only a minimal threat to the health of those who have it, use in amalgam also contributes to a gradual accumulation of the toxic element in our environment,” notes the United Nations. One more reason to give up.
Ecological impact of mercury
In aquatic ecosystems, birds and mammals that eat fish are among the animals most exposed to methylmercury because this metal tends to accumulate in living systems in a process known as bio-magnification.
Being very slow or impossible to dispose of, mercury accumulates in organisms at the top of the food chain, of which we are also part.
At high levels of exposure, the harmful effects of methylmercury on animals include abnormal behaviour, impaired reproduction, slow growth and development and, in the most egregious cases, death. It also tends to bind easily to compounds containing sulfur, which makes it an element capable of easily attacking the functionality of cells, enzymes and some essential amino acids.
The release of mercury into the environment is partly natural and partly man-made. In nature this element is emitted through volcanic eruptions and forest fires, being present in traces both in the organic and inorganic components.
However, the bulk of the release of mercury is due to human activities involving the combustion of coal and hydrocarbons (energy production, domestic heating, disposal of mercury-containing waste) together with some industrial processes and mining activities related to the extraction of mercury, gold and other metals in which it is present.
Human activities have increased the total concentrations of mercury in the atmosphere by about 450% compared to natural levels, with an increase of about 20% in 2015 compared to 2010 values (Global Mercury Assessment, 2018).
Health prevention and control
It is possible to come into contact with mercury by ingesting large quantities of foods that contain it, such as large fish, crustaceans and molluscs, but also by living near mines or factories that release it into the water and air. Accidental breakage of mercury-containing tools can cause toxic evaporation and inhalation. Prolonged exposure to high levels of mercury can damage the brain, heart, kidneys, lungs and immune systems of people of all ages. In contrast, high levels of methylmercury in the blood can impair the development of children and pregnant women (you will find more information here WHO).
Tuna fish
As far as diet is concerned, the European Food Safety Authority recommends consuming fish 2-3 times a week, avoiding preferring species potentially richer in methylmercury (swordfish, tuna, sharks, etc. ). Before arriving on our tables, the EC Regulation n.1881/2006 provides for strict controls to verify the levels of mercury in food in all the phases that precede the marketing of the product.
The Mercury laws
Following a serious environmental and health disaster known as the Minamata case (Japan, the 1950s), during which large quantities of industrial mercury were released into the sea, many countries adhered to the 2013 International Minamata Convention. With this agreement, the signatory members (including Italy) undertake to control and reduce the use of mercury in numerous products and industrial processes, leading to its total ban in 2020.
In parallel to this, optimizing the disposal of mercury-containing waste and safety protocols in the event of direct contact would help contain the risks associated with this substance.
Silver mercury uses
Silver mercury is one of the widely used elements in various fields of industry because of the properties that make it good for these industries, and among those uses are the following:
Manufacture of thermometers due to their survival in the liquid state for different temperatures, how much it has the property of expansion and contraction under the influence of high and low temperature.
Manufacture of radiant and incandescent lamps as a result of passing electric current over mercury vapour.
In the field of medicine, mercury is used in the preparation of antiseptics known as micro chrome, in lubricants used to treat kidney diseases, and in the manufacture of dental fillings known as amalgam.
The cosmetics industry, especially those that give the skin whiteness and luster.
Extraction of gold from its natural ores.
Preparation of compounds used in the detection of organic compounds in the form of Hg2SO4, ie mercury sulfate.
Batteries in the form of mercury oxides.
Manufacture of paints, especially red ones. Mercury-containing paint is also used to paint the walls of ships to eliminate fungi and marine bacteria that grow on them.
Manufacture of lamps, electrical appliances, paper and leather tanning.
Manufacture of alloys containing a mixture of mercury and one or more elements used in manufacturing processes.
Mercury Hazards
Vapour inhalation is the main route of the entry of metallic mercury into the body. About 80% of inhaled mercury vapour is absorbed into the lungs (alveoli). Gastrointestinal absorption of metallic mercury is minimal (less than 0.01% of an ingested dose). Penetration of mercury is also possible. Metal under the skin resulted from an accident (such as a broken thermometer).
The main routes of entry for inorganic mercury compounds (mercury salts) are the lungs (atomized mercury salts) and the gastrointestinal tract, in the latter case, absorption is often the result of accidental or voluntary ingestion, with it estimated that 2 to 10% of ingested mercury salts is absorbed through the intestines.
It is possible for the skin to absorb metallic mercury and some of its compounds, although the rate of absorption is low after entering the body. Hence, the presence of metallic mercury lasts for a short time in a metallic form, which explains its penetration into the blood-brain barrier, as it quickly oxidizes metallic mercury in the blood and tissues to Hg2 + mercury ion, which is fixed in proteins in the blood. Inorganic mercury is also distributed between the plasma and red blood cells. The kidneys and brain are the sites of deposition after exposure to metallic mercury vapours, and the kidneys after exposure to inorganic mercury salts.
Symptoms of acute poisoning include lung irritation (chemical pneumonia), which may lead to acute pulmonary edema. It also affects the kidneys. Acute poisoning often occurs as a result of accidental or voluntary ingestion of mercury salt.
This leads to acute inflammation of the gastrointestinal tract, quickly followed by renal failure due to necrosis of the proximal coiled tubules.
The severe, chronic mercury poisoning that places like Almaden faced until the early 20th century, which caused renal, digestive, mental, and neurological disorders and ended in cachexia, has been eradicated by preventive measures.
However, chronic “intermittent” poisoning can still be detected interspersed Active poisoning between periods of latent poisoning among mercury miners. In latent periods, symptoms move to a degree that can only be seen upon careful investigation; Only neurotic manifestations persist in the form of profuse sweating, ecchymosis, and to some extent, emotional instability.
A case of “microuria” characterized by functional neurosis (recurrent hysteria, neurasthenia, and mixed forms), cardiovascular capacity, and secretory neurosis of the stomach has also been described.
Gingivitis is the most common gastrointestinal disorder in cases of mercury poisoning, favoured by poor oral hygiene and accompanied by a metallic or bitter taste in the mouth.
Ulcerative membranous stomatitis is less common and usually found in people already suffering from gingivitis who have accidentally inhaled mercury fumes. Stomatitis also begins with the subjective symptoms of gingivitis with increased salivation (mercury) and tongue covering.
Eating and drinking results in a burning sensation and discomfort in the mouth. The gums become increasingly inflamed and swollen. Also, ulcers appear, and spontaneous bleeding occurs. In severe cases, there is a high fever, inflammation of the submandibular ganglia, and foul-smelling breath. Alveolar periostitis is also noted.
There may be a bluish line at the edge of the tooth from the gums, particularly near the affected areas; however, it is rarely encountered in people without teeth, as a dark grey point pigmentation of the oral mucosa has also been observed – the vestibular side of the gums (usually in the lower jaw), the palate and even the insides of the cheeks.
Recurrent periodontitis affects the tissues supporting the teeth, and in many cases, the teeth must be extracted or simply fall out. Other gastrointestinal disorders encountered in mercury poisoning include gastritis and gastroenteritis.
Non-specific pharyngitis is relatively common. A rare manifestation is Kussmaul’s pharyngitis, which presents as a bright red discolouration of the pharynx, tonsils and soft palate with subtle arborization. Neurological involvement may occur with or without gastrointestinal symptoms and may develop in line with two main clinical pictures:
A subtle intention tremor that is reminiscent of those encountered in people with multiple sclerosis.
Parkinson’s disease with resting tremors and decreased motor function; usually, one of these two conditions predominates in the overall clinical picture, which may be further complicated by pathological irritability and excessive mental activity (mercurial erethism).
Mercurial Parkinson’s disease presents with a staggering, unsteady gait, lack of reflexes to restore balance and hypotonia; Vegetative symptoms are considered mild with mask-like faces such as drooling; however, Parkinson’s disease is usually encountered in milder forms, particularly young Parkinson’s disease.
The symptoms frequently encountered are similar to those presented by people with multiple sclerosis, except that there is no nystagmus and that the two conditions have different serotypes and clinical courses. The most striking feature is tremor, which is usually a late symptom but may develop before stomatitis.
The tremor usually disappears during sleep, and although sudden generalized spasms or spasms occur, however, it always increases under emotional stress, a distinctive feature that provides a strong basis for the diagnosis of mercury poisoning.
Tremor appears especially in situations where the patient feels embarrassed or ashamed; He will often have to eat in solitude; for otherwise, he would not be able to raise the food to his lips, and in its most severe form, the tremor may invade all voluntary muscles and be continuous, cases still occur in which the patient must be strapped to prevent him from falling out of bed, such cases also show sufficient collective graceful movements To awaken the patient from his sleep.
Safe storage methods for mercury products
- Place these products in large, well-sealed containers and place them out of reach.
- Attempt to place these products near materials that have the ability to absorb oil; In order to prevent product breakage.
- Try to put a label on which all the contents of the box are written, with a strong warning not to open it.
- Place the product in places that are difficult to reach and out of the hands of children while taking care to keep it in its original packaging and dispose of it properly and safely.
Related Articles:
- Zinc: Chemical Element Properties, Formation and Uses
- Platinum: All you need to know about the Strong element
- Manganese: What you should know about it


Leave a Reply